Introduction
What is Quality Control?
Quality Control (QC) is focused on identifying defects in products or manufacturing outputs. The goal of QC is to ensure that the product meets certain specified requirements by inspecting and testing the final product (or its components).
Within most quality management systems (e.g. ISO 9001:2015), QC is the process by which non-conforming manufacturing outputs are prevented from being shipped to customers. For example:
- For purchased components, inspections may be made to confirm that the vendor has provided the promised material to the specifications required.
- For manufactured assemblies, there might be inspections or, as for the case of electronic assemblies, hardware validation, testing, or calibration processes involved.
For manufactured outputs, quality control occurs after production and is reactive to defect and may include steps to correct defects.
What is Quality Assurance?
Quality Assurance (QA) is focused on preventing defects by improving and ensuring the quality of processes used to develop and deliver a product or service. The goal of QA is to establish a framework of standards, procedures, and methodologies to ensure that quality requirements are met consistently.
Quality Assurance occurs before production and is an ongoing, proactive activity. It could be said that QA is an “outer process” that is used to develop and improve the other processes in an organization. Among them:
- Training
- Manufacturing
- Quality Control
- Auditing and Compliance
Quality Control Scenarios
There are several situations where material may need to enter the quality control process for review. For example:
- When an assembly is built, the finished goods from that process may need to go directly into an inspection or validation process. Builds might initiate and end-of-line test. Aligni can automatically move the finished assembly inventory into a quarantine location and create a quality control record (QCR) to manage and document the process.
- Purchasing might want to initiate visual inspection or non-destructive testing for machined parts. This might be justified if, for example, usage of the material comes at a great cost and any defective material would generate costly scrap. Aligni can automatically move purchased material into quarantine and create a QCR for this as well.
Aligni Quality Control
Aligni’s quality control implementation is designed to accomplish three objectives:
- Enhance collaboration – The exercise of quality control often involves participation from several groups: production, engineering, testing. Providing an excellent user experience helps encourage engagement and improve quality and efficiency.
- Document process outcomes – in particular: inspection and condition assessment, defect root cause analysis, and material disposition
- Aggregate results for analytics – A database approach is crucial to aggregating results and organizing them into meaningful measurements.
We feel that there is great value in integrating this functionality into the same system where the item master, engineering change management, build management, procurement, and inventory management exist.
There are a number of settings available to organizations to customize the workflow for the products you sell, the processes you follow, and the analytics you need. These settings are described at the end of this page.
We recommend that your team review the process and try to configure your settings soon so that your data is as consistent as possible.
For more information please visit our Quality Control Reference Documentation.
Quality Control Under ISO 9001:2015
ISO 9001:2015 is the most current and relevant version of the standard for quality management put forth by the International Standards Organization. It establishes the benchmark that organizations can use when defining their own quality management system. One truly remarkable aspect about the standard is that it is defined and genuinely usable within the ‘context of the organization,’ meaning that it is equally applicable for very small companies as well as enormous multi-national enterprises.
Another remarkable aspect is that, while it stands as a formal document establishing practices to build products of exceptional quality, the guidance is very approachable and, in fact, common sense. Consider, for example, the requirements under Section 8.1: Operation Planning and Control. Paraphrasing the standard (but not by much!), this section states that the organization must:
- Figure out the requirements for products and services. That is, determine what defines a product or service that “isn’t broken”.
- Define criteria for acceptance. Or, put another way, define criteria for how something would be considered broken.
- Figure out what resources you need to build stuff that isn’t broken.
- Implement processes for #2 (figuring out when something is broken).
- Measure, document, and store the records that demonstrate that each item (or service) you ship to customers isn’t broken.
These are all things your organization should be doing anyway, right? But the challenge isn’t really identifying what you should be doing. Most people building a product or delivering a service already know what they should be doing to assure high quality output, but they may not know how to efficiently manage the process. This is why choosing the right software can make the difference between successful execution and burning time, burning money, and losing focus.
So let’s review a few of the provisions in ISO 9001:2015 where dedicated software can help your organization perform better and more efficiently.
ISO 9001:2015 Section 8.4 – Control of Externally Provided Processes, Products, and Services
Section 8.4 outlines the controls that the organization must have in place related to materials and services provided externally — that is, by a vendor or partner. It basically states that the organization must establish processes, evaluation criteria, and controls for these materials and services so that the products and services it delivers to customers are not affected by nonconformances of the vendor.
In other words, this section establishes that your organization is ultimately accountable for the failures of your vendors because it’s your responsibility to monitor and control those materials and services.
Aligni’s Quality Control functionality allows you to place a quality control gate at two crucial locations to help with this:
- When receiving purchased materials from vendors; and
- When receiving built assemblies from manufacturing partners
By using quality control and quarantine inventory locations appropriately, Aligni provides the software tools you need to support the requirements in section 8.4 so you don’t need to create ad-hoc processes from more generalized software such as spreadsheets and smart spreadsheets..
ISO 9001:2015 Section 8.5.2 – Identification and Traceability
This section establishes the need for your organization to appropriately identify material to ensure conformity. This is typically managed with identifying marks such as serial numbers, date codes, lot codes, and so on. Aligni’s inventory unit record allows you to store and track these attributes throughout the inventory’s history.
This section also establishes the need for the organization to identify the status of material throughout the quality control process and to retain this information to the extent necessary. Aligni’s approach to QCR binning, assessment, and disposition provides elegant support for this effectively and efficiently.
Keep in mind that the level to which traceability is required is up to the organization relative to the context in which it operates. The standard states that, when traceability is required, the documentation is required to support it. But it does not specify that every ISO 9001 organization have full traceability. The effort required to do this is often expensive and may not be justified.
ISO 9001:2015 Section 8.7 – Control of Nonconforming Outputs
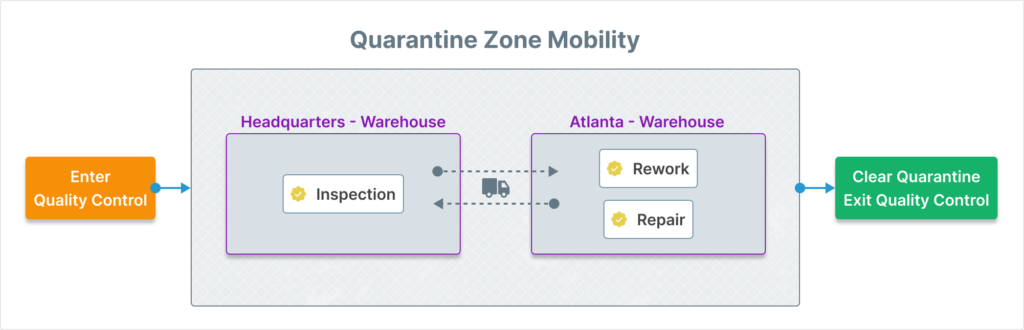
This provision (in addition to Section 8.6 – Release of Products and Services) is concerned with assuring that material under review is properly segregated (or quarantined) and that it is released only after the planned review process has completed.
To support the Quality Control process, Aligni warehouses support specialized areas (quarantine warehouse zones) for managing this segregation. Inventory may only move into a quarantine location with the creation of a QCR and may only exit quarantine by closing that QCR. This software support helps to make the physical segregation of inventory much easier to manage and document.
Additional Notes
ISO 9001 establishes the need to create processes. This inherently means that the organization needs to define what is required within the context of their organization. Aligni provides some of the infrastructure to support this but the organization still needs to provide the details of execution and additional procedures required to determine whether or not something is conforming.
Tips and Best Practices
Using the “QC Required” Attribute
Each item in your item master has a QC Required attribute that can be set to true or false. When false (the default value), purchases and builds do not prescribe any particular behavior for these items. They can be received to any standard warehouse zone or quarantine zone. When received to a quarantine zone, a QCR will be created, but this is not required.
When true, purchases and builds will enforce the rule that these items must be received to a quarantine zone and, therefore, a QCR will always be created. This drives the material into the quality control system for handling.
Some purchased components and most assembled items will likely require some level of quality control to meet quality standards and to assess vendor performance. We recommend that, when any doubt exists, to err on the side of caution and set the “QC Required” attribute for these items.
If only light quality control is required (for example, just a quick visual inspection), this can be managed quickly and easily with Aligni’s quality control system as well. The benefit is that even this light inspection is logged for the record and creates an audit trail.
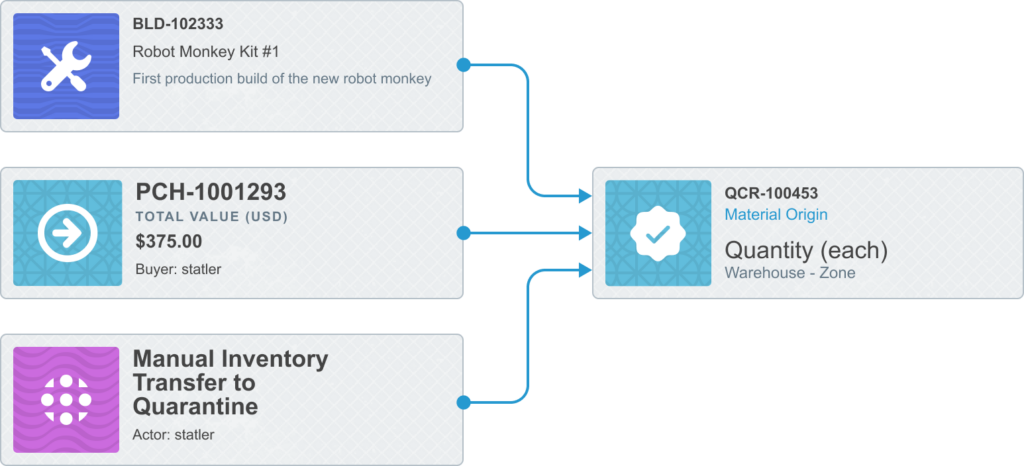
Furthermore, Aligni links the inspection (QCR) to the purchase or build record for easy reference. This link cannot be established later.
Splitting Partial Shipments
Aligni provides a means to handle situations where a batch of material needs to be split into multiple quality control batches.
Completed Assemblies are Shipped in Batches
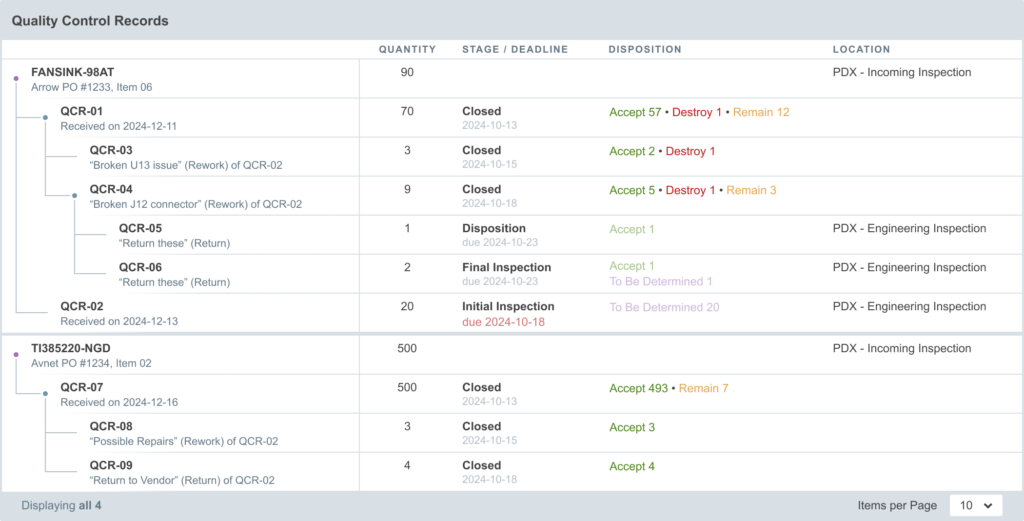
When a vendor ships multiple shipments of a single work order, it may be appropriate to finalize the full batch build, but receive the material in separate shipments. In this case, the build may be finalized and create a single QCR. In the triage state, use the “Split Inventory” feature to create one or more additional QCR for the split batches. This approach allows Q/C to proceed with the individual batches, but maintains the link to the origin build for reference.
Receiving Partial Shipments on a Purchase Order
When partial shipments are received on a purchase order, each of the individual receipts will create a new QCR for the corresponding material. While these QCR are not, themselves, linked together, they are linked to the same origin and will be shown on the purchase’s Quality tab.
Sharing the Load
Any QCR may be split in the triage state. This may be useful to split larger quantities of material among multiple team members. When QCR inventory is split in this manner, both QCR will exist at the same level of the “QCR tree” and will link back to the same parent.
Project Management in Quality Control
There are several approaches to project management within the Q/C team that could be deployed depending on your objective, resources, and throughput requirements. Here are just a few suggestions.
Reduce Triage Backlog
You can help keep triage population to a minimum by setting deadlines once a QCR is created and assigning QCR to individual personnel for accountability. Use “at-mentions” within the QCR discussion to notify your team of pending QCR.
Reduce Aging
Lingering QCR can create an extensive backlog and operational debt. By regularly reviewing the Quality Control index and sorting by “Age”, you can turn your focus to the oldest QCR and encourage your team to close them.
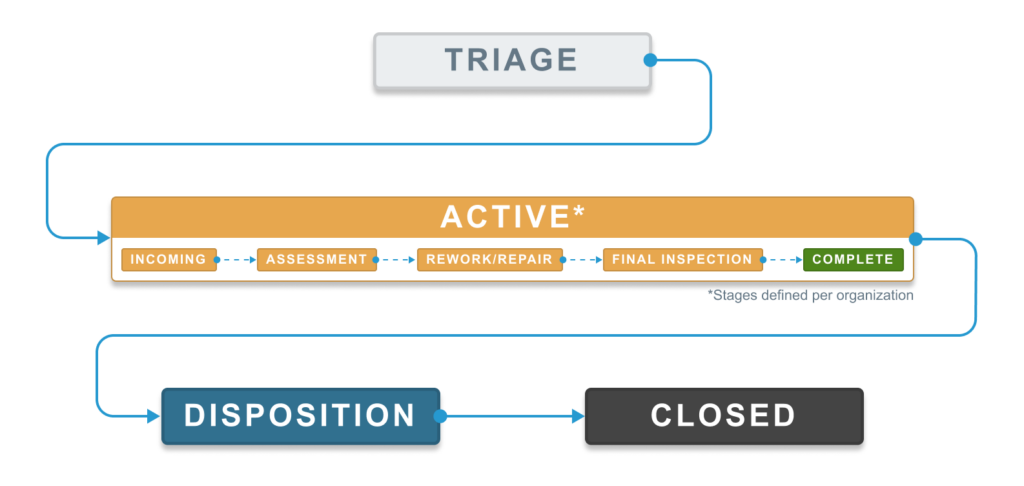
Establish Stages to Map to Internal Processes
Progress through the active state for QCR is left for each organization to define. While we provide one set of example stages, this is just a template to start from. We encourage each organization to define these stages according to their internal process requirements. Ideally, progress through the stages follows a typical path, but Aligni does not require or enforce any particular direction. Within the active state you’re allowed to set the stage to anything.
When sorting the QCR index by stage, the sort order within the active state follows the sort order that you’ve established in the organizational settings for the stages you’ve defined.
Review Quality Results Regularly
We recommend routine reviews of your quality control outcomes. Depending on the size and activity of your organization, it may be appropriate to do this on an annual, quarterly, or even weekly basis. As an agenda for this review, you may want to keep track of critical vendors, materials, or assemblies and review the QC outcomes related to those items with your team.
You can extend these discussions to involve your vendors to provide feedback on your internal processes and their performance. A good vendor will appreciate getting this objective feedback. A great vendor will ask for it!